-40%
CONTEC ECG1200G Digital 12 lead EKG Electrocardiograph PC Sync software 12chanel
$ 316.27
- Description
- Size Guide
Description
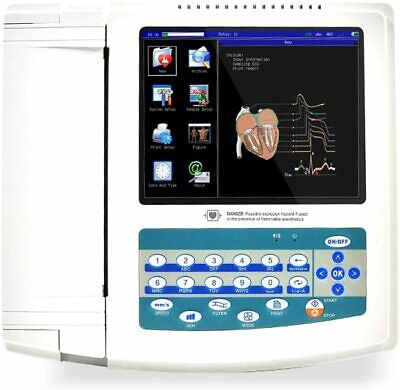
PORTABLE 12 CHANNEL ECG/EKG MACHINETOUCH SCREEN
Tips:
USA Buyer:
ship from USA warehouse
. Fast delivery (2-5 days) ;
Other Buyer :
ship from China Warehouse
. 1-6 weeks delivery
Introductions
ECG1200G twelve channel ECG is such a kind of electrocardiograph,which samples 12 leads ECG signals
simultaneously and prints out the ECG waveforms with thermal printing system. Its functions are as
follows: Recording and displaying ECG waveforms in auto/manual mode; Auto-measuring and auto-
diagnosing the ECG wave parameters; Prompting the state of lead off and paper lack; Switching several
kinds of interface languages; Managing the case database.
Features
8"TFT Color LCD shows the working status and ECG waveform. Check the waveform before printing to save
record paper.
12 Leads ECG simultaneous gather, digital signal processing, to get the ECG waveforms of higher quality
through the AC, DFT and EMG filtering of the ECG signals.
Simultaneous display of 3/6/12 leads ECG waveform, and the state of printing mode, sensitivity, speed,
filter etc, to be easy for contrast diagnosis.
Multiply printing modes and formats, including 12*1, 6*2+1(rhythm lead), 6*2, rhythm 12, rhythm 10,
rhythm 8, rhythm 6, manual and freeze, etc. The length of printed wave can be adjusted and simultaneity
the function of periodic printing is offered to satisfy different applications.
The instrument has the functions of auto-analysis and auto-diagnosis of regular ECG waveform parameters,
provides measurement parameters such as HR, P-R interval, P Duration, QRS Duration, Q-T interval, Q-Tc,
P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1), etc and auto-diagnosis conclusion to reduce doctor's
burden.
AC/DC power supply. Built-in rechargeable lithium polymer battery. At the best DC state, it can standby
for 10 hours, print less than 3 hours, and print 300 pieces of ECG waveform to satisfy the need of visiting
patients and physical examination.
Built-in MSF, can store more than 1000 cases, convenient for doctor's case review and information statistics.
System provides Chinese/English/Spanish/Turkish/German/Portuguese/Kazakhstan/Russian operation interface
and can print out such language reports of the above.
System provides multiply printing speeds, including 5mm/s, 6.25mm/s, 10mm/s, 12.5mm/s, 25mm/s, 50mm/s,
and abstract conclusion graph printing.
Technical Specification
Environment conditions
Operation
a) Environment temperature: +5℃~+35℃
b) Relative humidity: ≤80%
Input way: Floating and defibrillation protection
Sampling precision: 12 bits
Lead: Standard 12 leads(model:BIP0057)
Patient leak current: <10μA
Input impedance: ≥50MΩ
Frequency response: 0.05Hz~150Hz(-3dB~+0.4dB)
Time constant: Time constant≥3.2s
CMRR: >60dB, >100dB( Adding filter)
EMG filter:25Hz/35Hz (-3dB)
AC filter:50Hz/60Hz (≥20dB)
Recording way: Thermal printing system
Specification of recording paper: 210mm(W)*20m(L) high-speed thermal paper
Measurement parameters: HR, P-R interval, P Duration, QRS Duration, T Duration, Q-T interval, Q-Tc, P A*is, QRS A*is, T A*is, R(V5) , S(V1) , R(V5)+S(V1).
Product safety type: Class I CF applied part, which includes defibrillation and pacing protection circuit.
Size: 340mm(L)*320mm(W)*85mm(H)
Net Weight: 3.2Kg
Accessories
Standard:
ECG lead wire 1 set
Limb electrode clamp 1 set
Chest electrode 1 set
Power cord 1 piece
Ground wire 1 set
Thermal recording paper 1 set
User manual 1 set
ECG data line
Optional:
External ink jet printer
3G and WIFI
(GB only)on request for other Language
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923,and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved